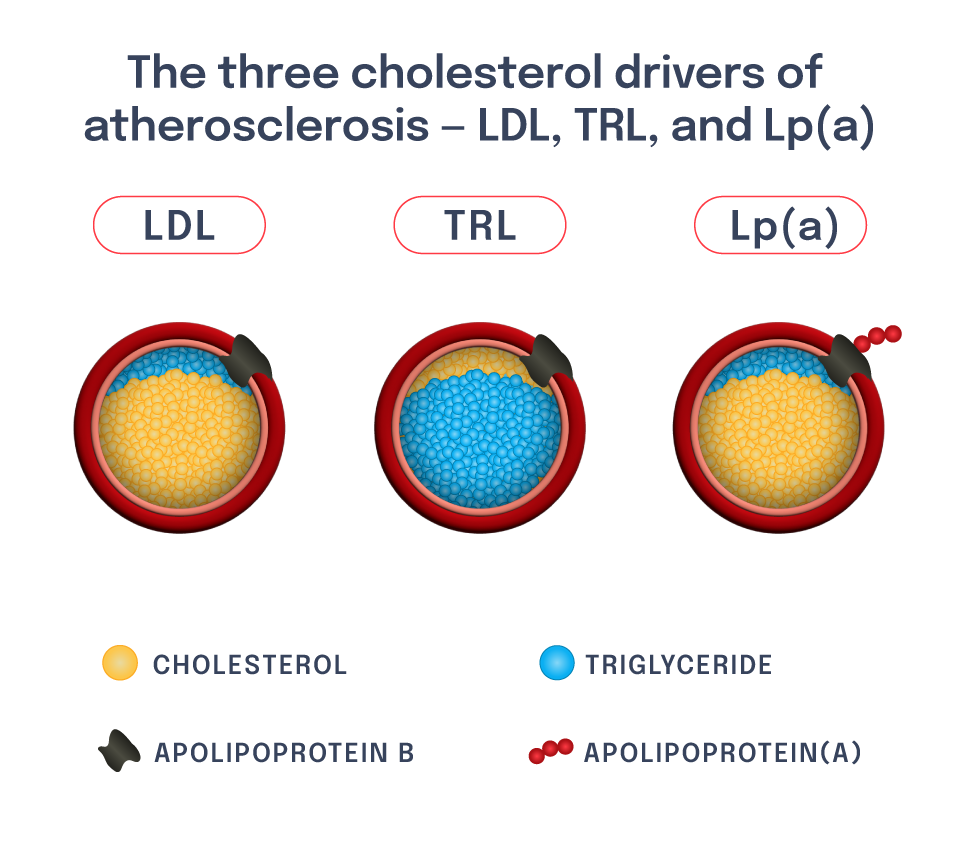
The LPA gene regulates lipoprotein(a), or Lp(a), which is a liver-derived lipoprotein that circulates in blood. It is very similar to an LDL particle, though it has another protein attached to it called apolipoprotein(a), or apo(a). High blood levels of Lp(a) contribute to risk of ASCVD, including ASCVD outcomes such as heart attack.
Patients with high Lp(a) are distinct from those with high LDL-C – though both are at risk of ASCVD, there is a low correlation between blood LDL-C levels and blood Lp(a) levels.
Both human genetics studies and human pharmacologic studies have validated the potential efficacy and safety of an Lp(a)-reducing medicine. DNA variants that cause increased circulating Lp(a) are among the strongest inherited drivers of risk for ASCVD as well as certain heart valvular diseases (e.g., aortic stenosis). By contrast, naturally occurring loss-of-function mutations in one or both copies of the LPA gene are associated with protection from these conditions and no apparent serious adverse health consequences.
In addition, recent human pharmacologic studies of investigational therapies targeting LPA expression in the liver have demonstrated the ability to potently lower circulating Lp(a) concentrations by greater than 80%. The potential for these medicines to lower the risk of recurrent ASCVD events in patients with high Lp(a) is being tested in ongoing cardiovascular outcomes trials.
We believe that these prior studies provide substantial evidence for the potential utility of a single-course medicine to lower Lp(a) in a patient population with both high risk and high unmet need. In collaboration with Eli Lilly, we are working toward developing a novel gene editor to target the LPA gene through this preclinical-stage program.
Our pipeline