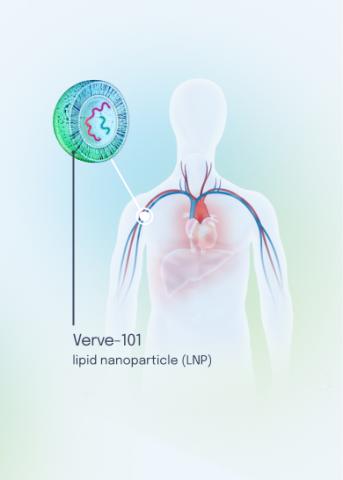
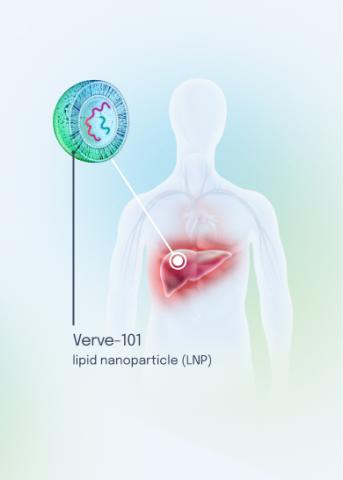
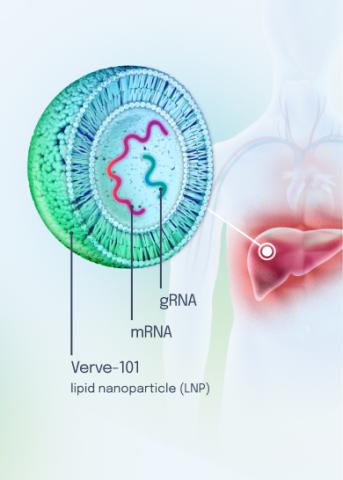
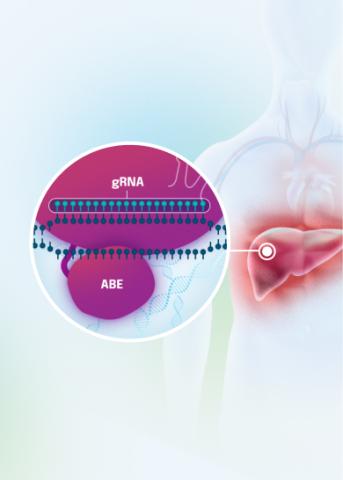
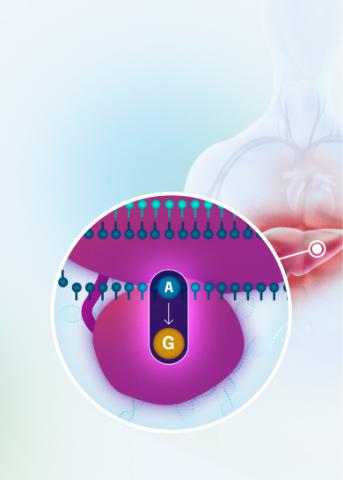
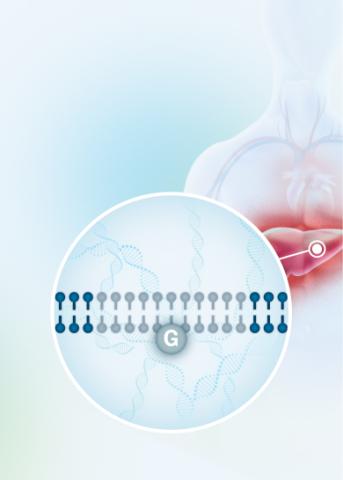
Our VERVE-102 product candidate targets the PCSK9 gene, a key regulator of cholesterol in the liver. Disrupting PCSK9 protein production may lead to durable reductions in low-density lipoprotein cholesterol (LDL-C).
VERVE-102 is a novel, investigational in vivo base editing medicine that is administered as an intravenous (IV) infusion over the course of a few hours. We are utilizing our internally developed GalNAc-LNP to deliver a base editor targeting the PCSK9 gene to the liver.
VERVE-102 is currently being evaluated in our Heart-2 Phase 1b clinical trial in patients with heterozygous familial hypercholesterolemia (HeFH) and premature coronary artery disease (CAD), two populations that require deep and durable reductions of LDL-C levels in the blood for an extended period of time.
Heart-2 Phase 1b Clinical Data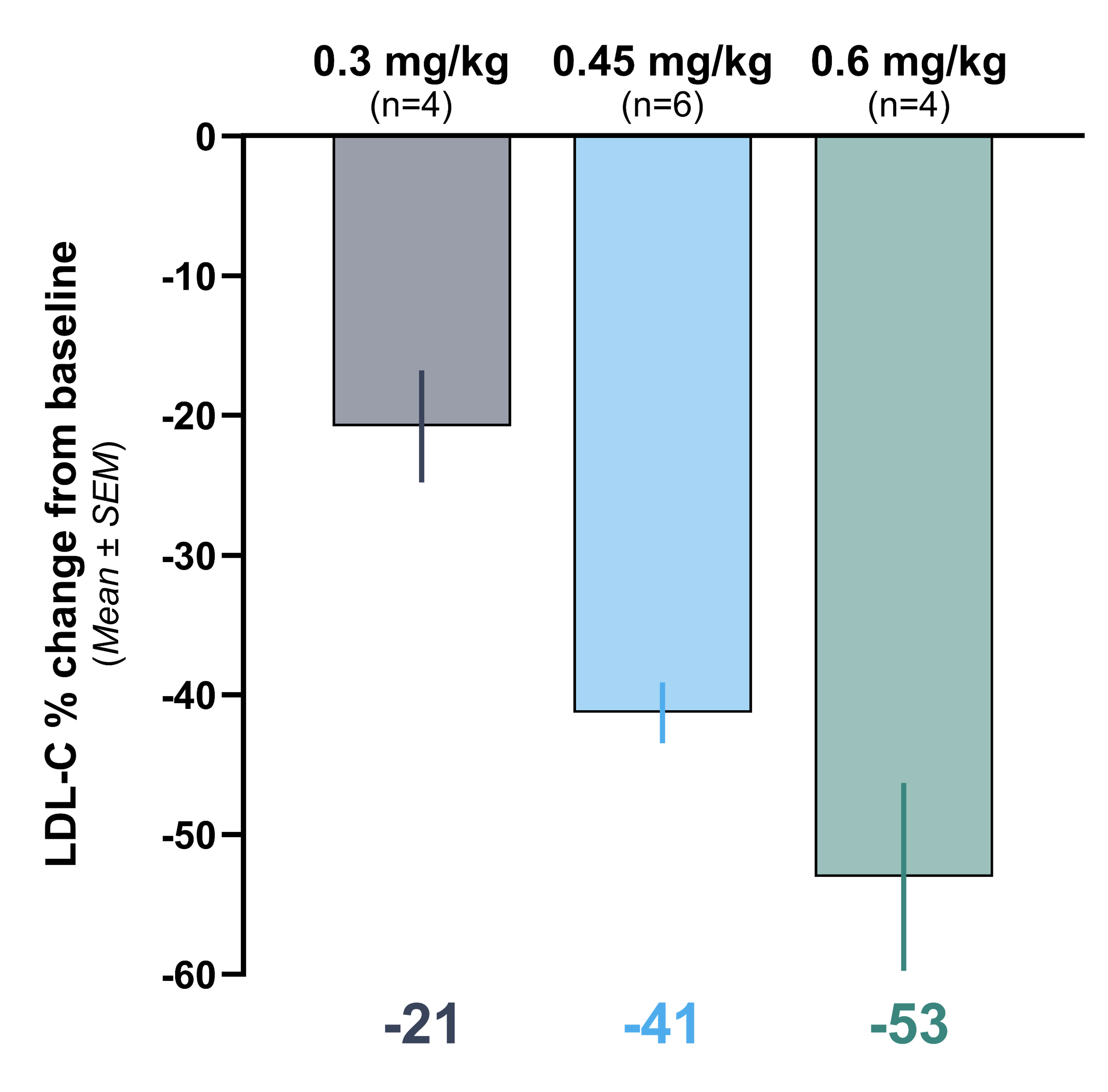
Initial data from the Heart-2 clinical trial announced on April 14, 2025 showed that VERVE-102 was well-tolerated, with no treatment-related serious adverse events (SAEs) and no clinically significant laboratory abnormalities observed. A single infusion of VERVE-102 led to dose-dependent decreases in blood PCSK9 as well as blood LDL-C, with mean reduction in blood LDL-C of 53% and maximum reduction of 69% observed in the 0.6 mg/kg dose cohort.
Graph showing time-averaged mean percent change in blood levels of LDL-C, at least 28 days after receiving VERVE- 102
- Cohort 1 (0.3 mg/kg) mean LDL-C reduction was 21%
- Cohort 2 (0.45 mg/kg) mean LDL-C reduction was 41%
- Cohort 3 (0.6 mg/kg) mean LDL-C reduction was 53%
Click here for the initial data presentation
We believe our VERVE-102 product candidate has the potential to treat a broad population of patients and plan to take a stepwise approach for clinical development, starting with the HeFH population before expanding to larger patient groups with established atherosclerotic cardiovascular disease and premature CAD. Following an evaluation of final clinical data from the dose escalation portion of the Heart-2 trial, we plan to initiate a Phase 2 clinical trial of VERVE-102.